Under Pressure Part 2
Just The Facts
DOFPro Team

Under Pressure
Under Pressure Part 2
- Atmospheric Pressure
- Barometric Pressure
- Gauge Pressure
- Absolute Pressure
- Vacuum Pressure
Under Pressure Part 1
- What pressure is
- How you use it
- How you measure it
Absolute, Atmospheric, and Gauge Pressure
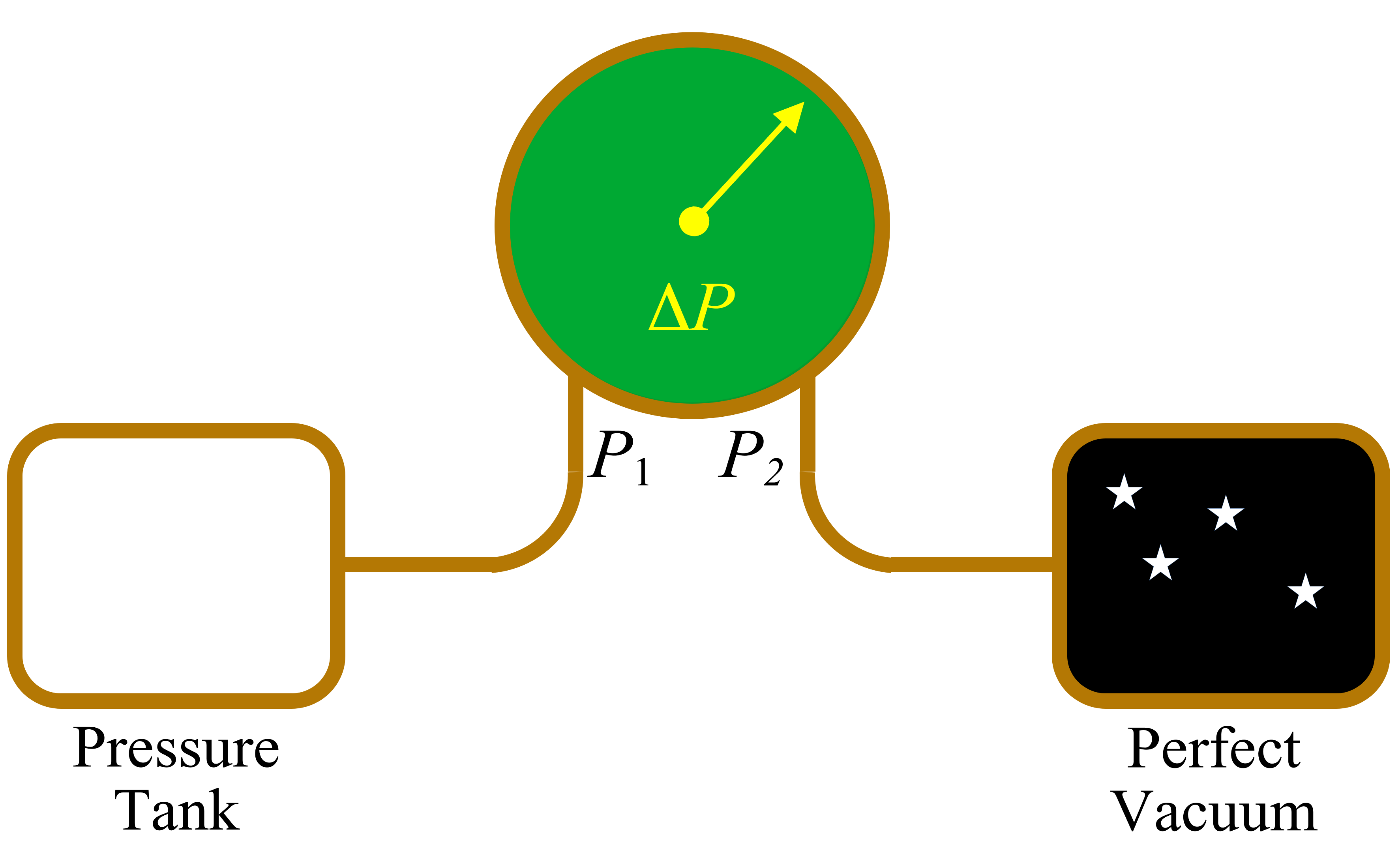
\(\Delta P = P_1 - P_2 = P_\mathrm{absolute}=P_\mathrm{abs}\)
e.g., \(30\ \mathrm{psia}\)
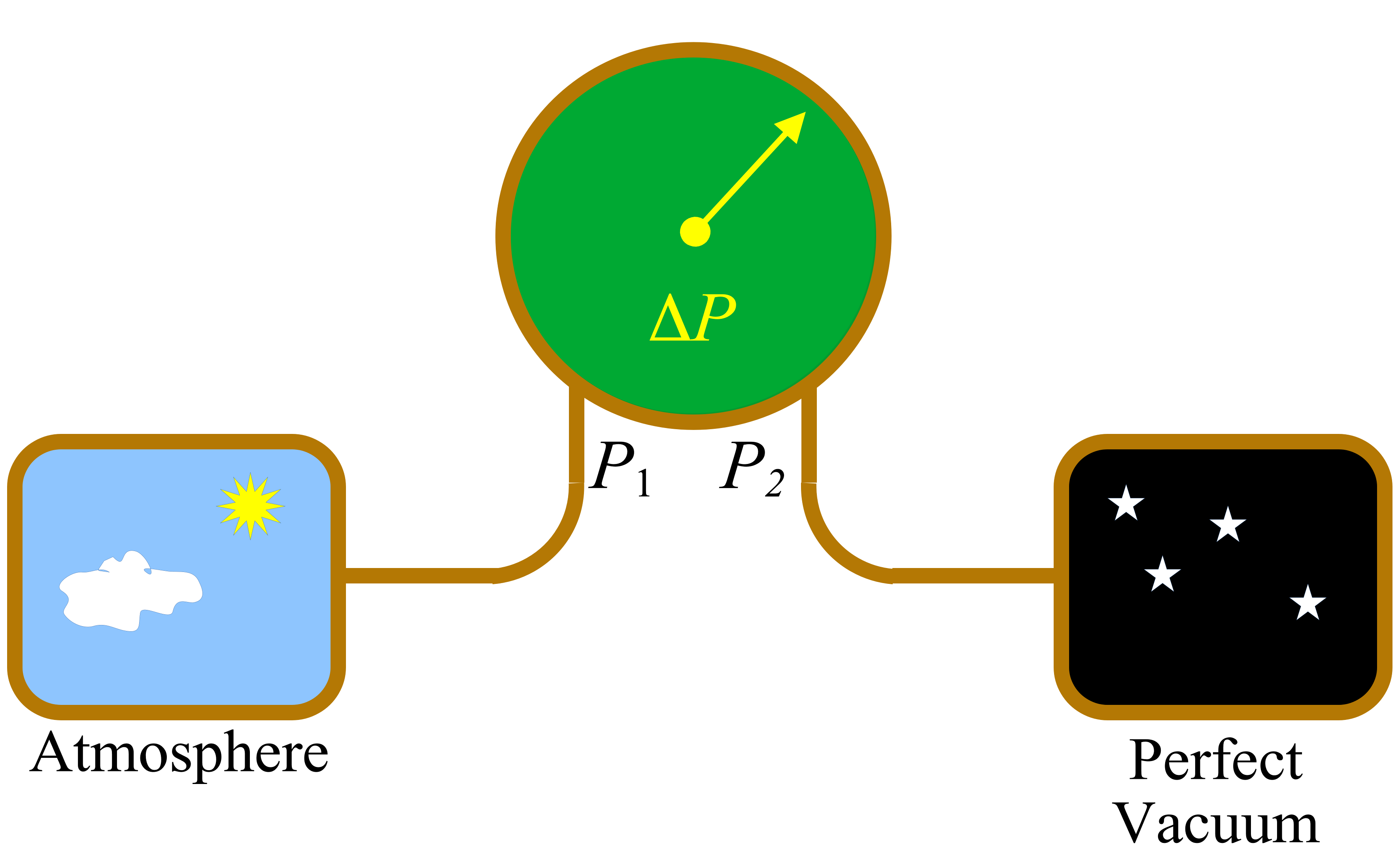
\(\Delta P = P_1 - P_2 = P_\mathrm{atmosphere}=P_\mathrm{atm}\)
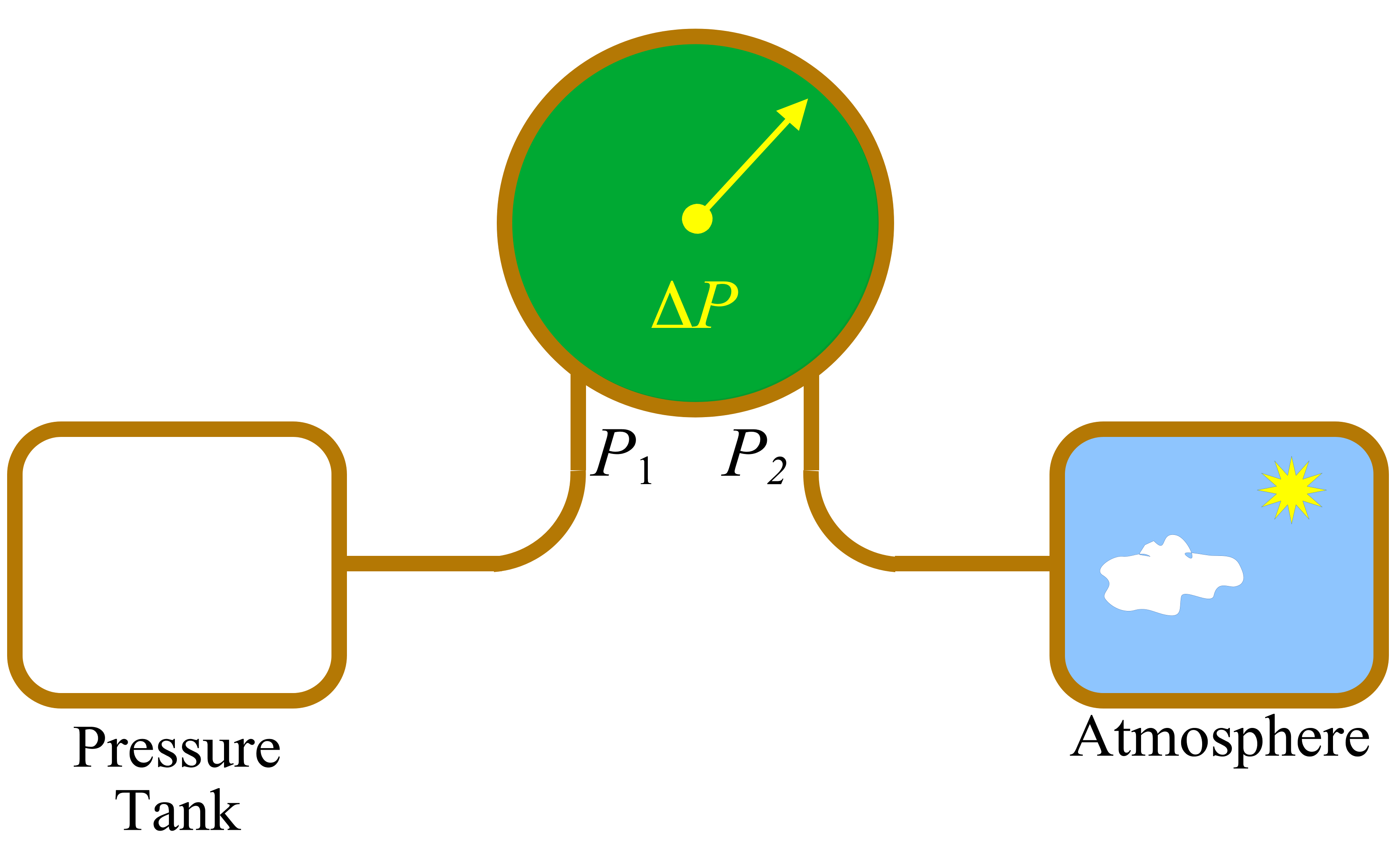
\(\Delta P = P_1 - P_2 = P_\mathrm{gauge}=P_\mathrm{g}\)
e.g., \(14.7\ \mathrm{psig}\)
Calculating Abs., Atm, Gauge Pressures
\(P_\mathrm{abs} = P_\mathrm{g}+P_\mathrm{atm}\)
\(P_\mathrm{g} = P_\mathrm{abs}-P_\mathrm{atm}\)
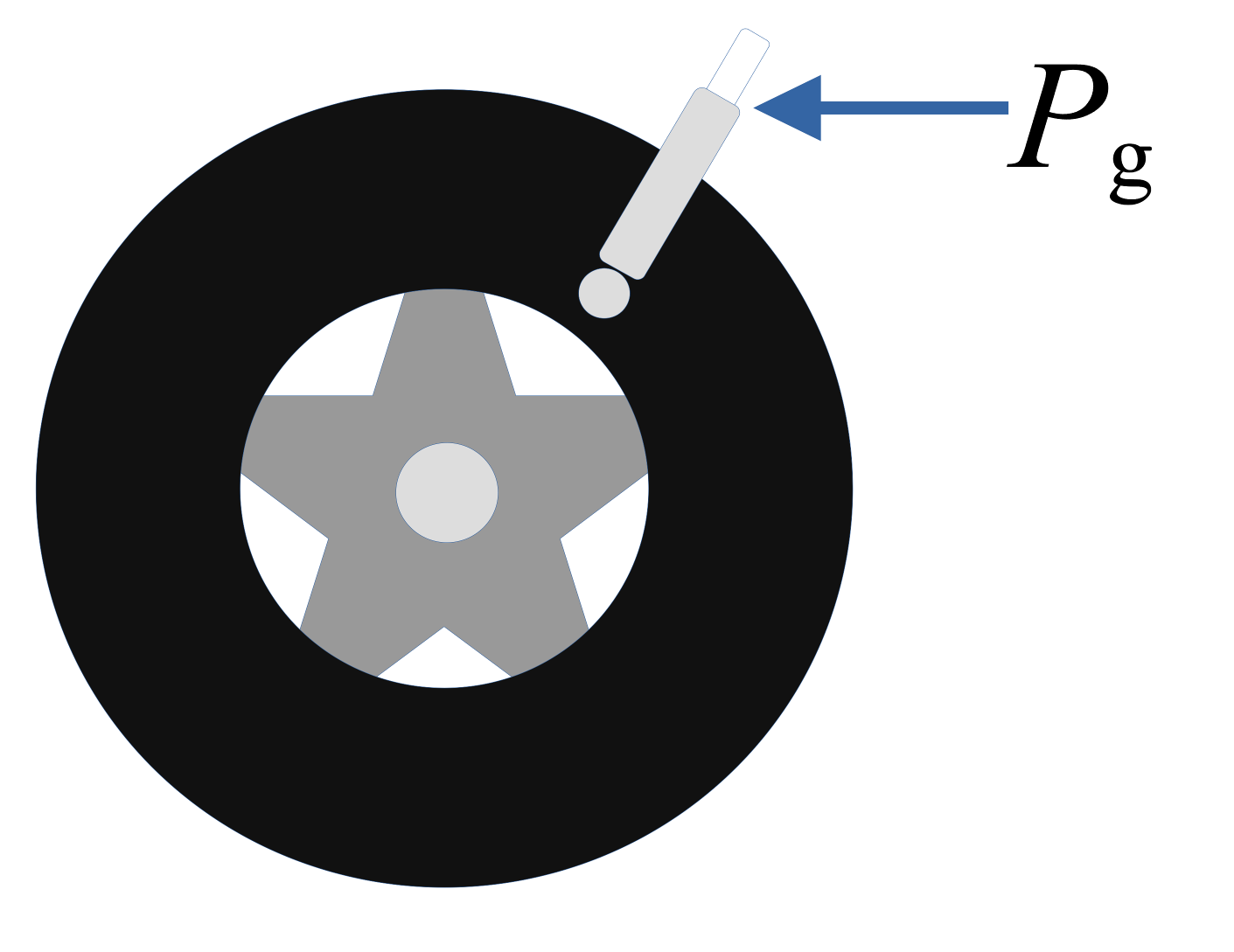
- Absolute tire pressure gauge
- Atmospheric pressure gauge
- Weather report and local altitude
Barometric and Atmospheric Pressure
- Absolute pressure gauges – Easy, Absolute tire pressure gauges – Very Hard
- Atmospheric pressure gauges – a decent cell phone and an air pressure app
- Weather report – Easy, Local altitude – Use GPS
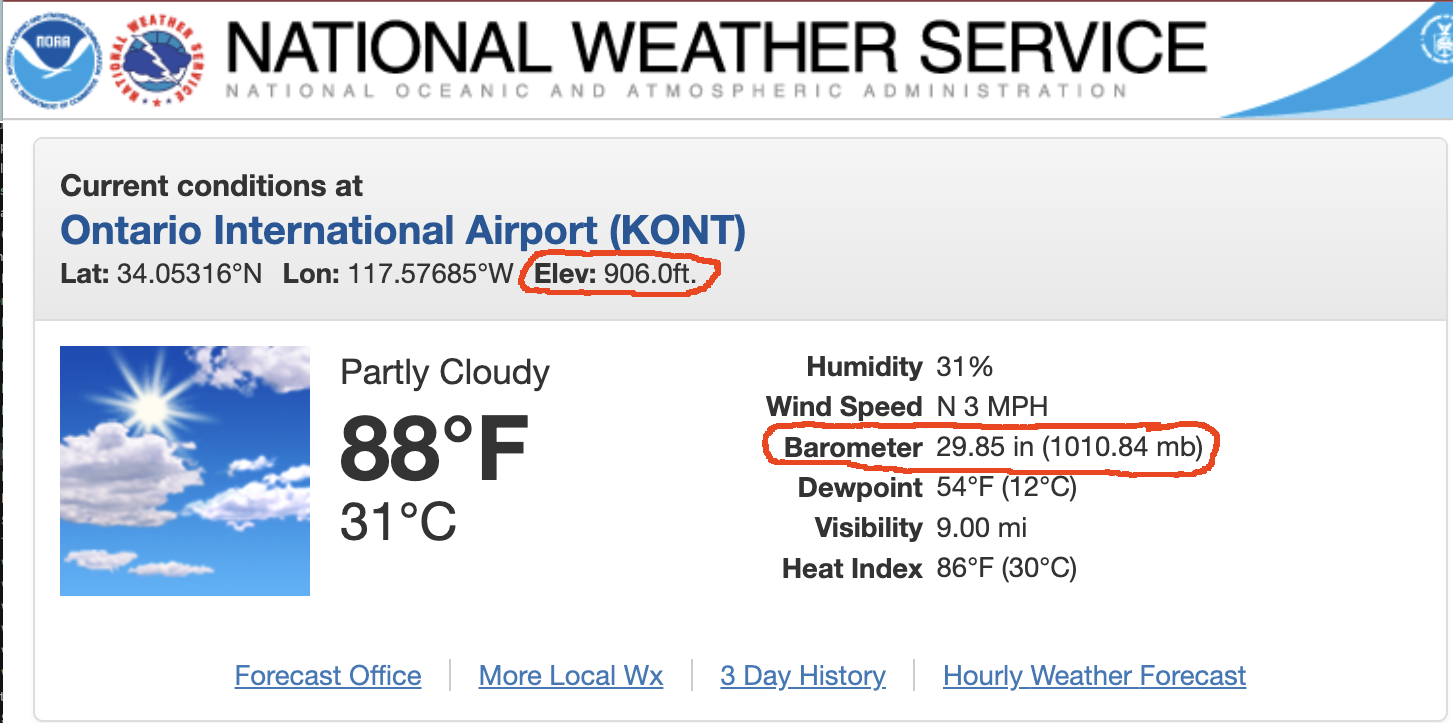
Weather report gives barometric, not atmospheric pressure.
Barometric Pressure
The barometric pressure is the local atmospheric pressure adjusted for altitude to sea level.
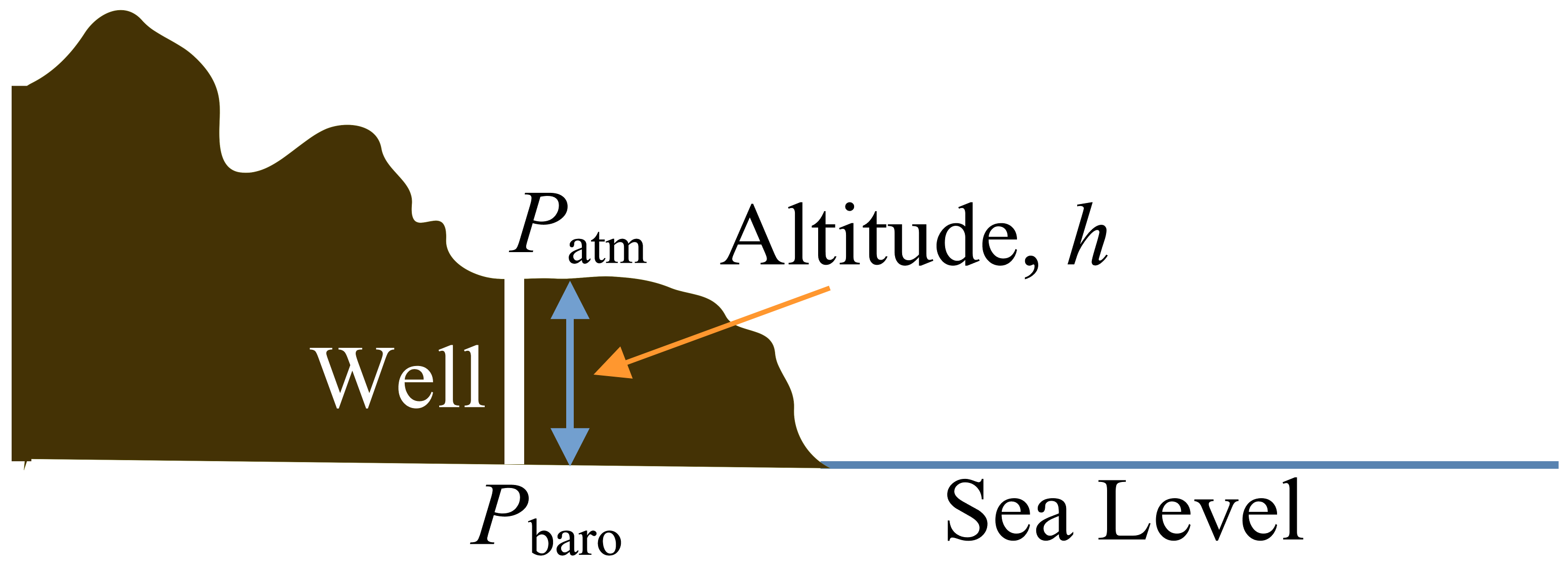
\[P_\mathrm{baro}=P_\mathrm{atm}(1-2.25577 \times 10^{-5}h)^{5.25588}\]
\[P_\mathrm{atm}=P_\mathrm{baro}(1-2.25577 \times 10^{-5}h)^{-5.25588}\]
with \(h\) in meters.
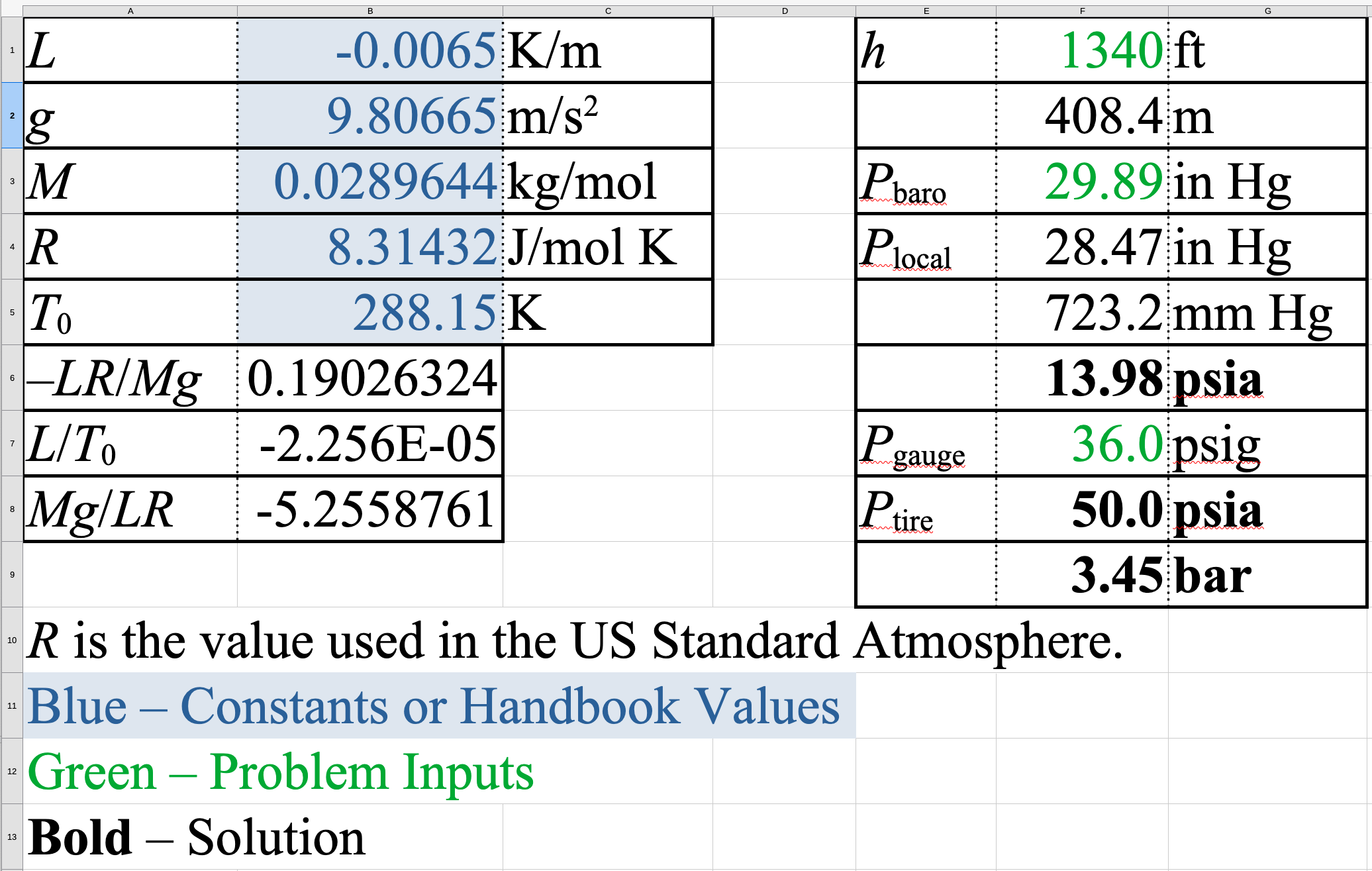
Example
Measure bicycle tire pressure in Claremont, CA, USA
\(P_\mathrm{baro} = 29.89\ \mathrm{inHg}\)
\(h_\mathrm{Claremont} = 1340\ \mathrm{ft}=408.4\ \mathrm{m}\)
\[\begin{aligned} P_\mathrm{atm} & =P_\mathrm{baro}(1-2.25577 \times 10^{-5}h)^{-5.25588} \\ & =29.89\ \mathrm{inHg}(1-2.25577 \times 10^{-5}\cdot408.4)^{-5.25588} \\ & = 28.47\ \mathrm{inHg}= 723.2\ \mathrm{Torr}= 13.98\ \mathrm{psia} \approx 14.0\ \mathrm{psia} \end{aligned}\]
\(P_\mathrm{g} = 36.0\ \mathrm{psig}\)
\[\begin{aligned} P_\mathrm{abs} & = P_\mathrm{g}+P_\mathrm{atm} \\ & =36.0\ \mathrm{psig}+14.0\ \mathrm{psia} = 50.0\ \mathrm{psia} =3.45\ \mathrm{bar} \end{aligned}\]
Spreadsheet
Vacuum Pressure
Background CC BY 3.0 link
- Any pressure below local atmospheric
- In psi it’s indicated by \(\mathrm{psiv}\).
- Negative of gauge pressure, e.g., \(P_\mathrm{g}=-7\ \mathrm{psig} \implies P_\mathrm{v}=7\ \mathrm{psiv}\)
- Common in vacuum distillation and other processes
High Vacuum
Background CC BY-SA 3.0 link
- Used in advanced materials processing
- Measured as absolute pressure
| Acronym | Name | Max P (Pa) | Min P (Pa) |
|---|---|---|---|
| HV | High Vacuum | \(10^{-1}\) | \(10^{-5}\) |
| UHV | Ultrahigh Vacuum | \(10^{-5}\) | \(10^{-10}\) |
| XHV | eXtrememly High Vacuum | \(10^{-10}\) | \(<10^{-10}\) |
The Takeaways
- Absolute, gauge, and atmospheric pressure are related by \(P_\mathrm{abs} = P_\mathrm{g}+P_\mathrm{atm}\).
- Barometric and atmospheric pressure are related through the Standard Atmospheric Model.
- The vacuum pressure is the absolute value of the gauge pressure below atmospheric.
- The High Vacuum (HV) range is from \(10^{-1}\) to \(10^{-5}\ \mathrm{Pa}\), Ultrahigh Vacuum (UHV) from \(10^{-5}\) to \(10^{-10}\ \mathrm{Pa}\), and eXtremely High Vacuum (XHV) is \(10^{-10}\ \mathrm{Pa}\) and below.
Thanks for watching!
The Full Story companion video is in the link in the upper left. The next video in the series is in the upper right. To learn more about Chemical and Thermal Processes, visit the website linked in the description to find previous and following videos in this series.
The DOFPro Team

