What Is A Mole
Just The Facts
DOFPro Team

Definition
- Mole = mass equivalent of the molar mass of a molecule
molar mass \(\mathrm{CO_2}\): \(44.010\)
gram-mol equivalent \(\swarrow\)
1 gram-mol \(\mathrm{CO_2} \rightarrow\) 44.010 grams
\(\searrow\) lb-mol equivalent
1 lb-mol \(\mathrm{CO_2} \rightarrow\) 44.010 \(\mathrm{lb_m}\)
How many different types of moles are there?
- 15 mass units on N.I.S.T website \(\rightarrow\) at least 15 types of moles
- range: carat-mole — pennyweight-mole
- Most common moles are:
- \(\text{g-mol}\) or \(\text{mole}\)
- \(\text{kg-mol}\) or \(\text{kilomole}\)
- \(\text{metric-ton-mole}\) or \(\text{tonne-mole}\)
- \(\text{lb-mol}\)
- \(\text{ton-mole}\)
Using a Mole
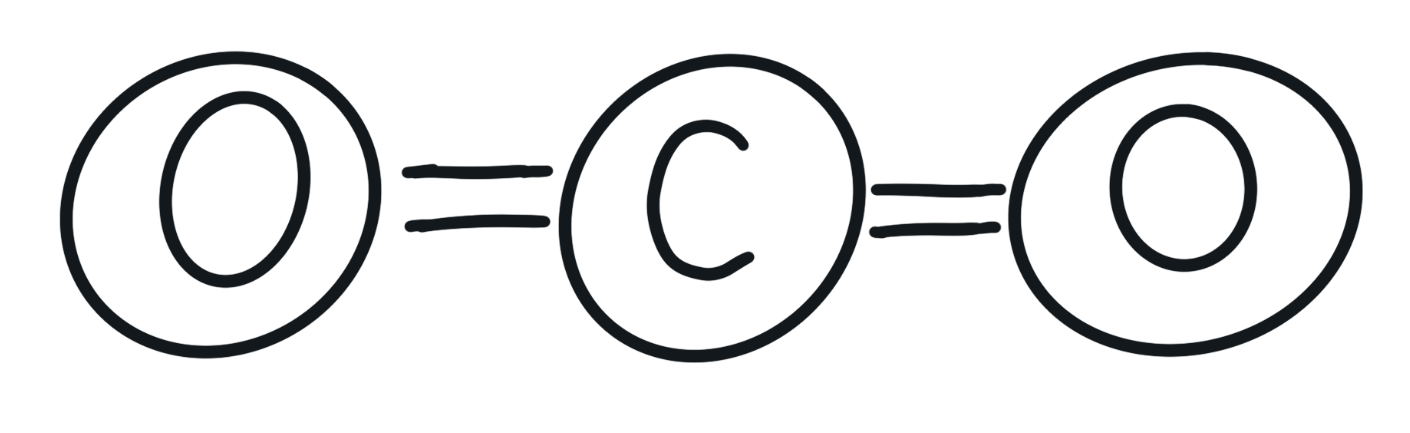
Start with a balanced chemical equation.
\(\mathrm{C} + \mathrm{O_2} \rightarrow \mathrm{CO_2}\)
To make \(100\ \mathrm{lb_m}\) of \(\mathrm{CO_2}\):


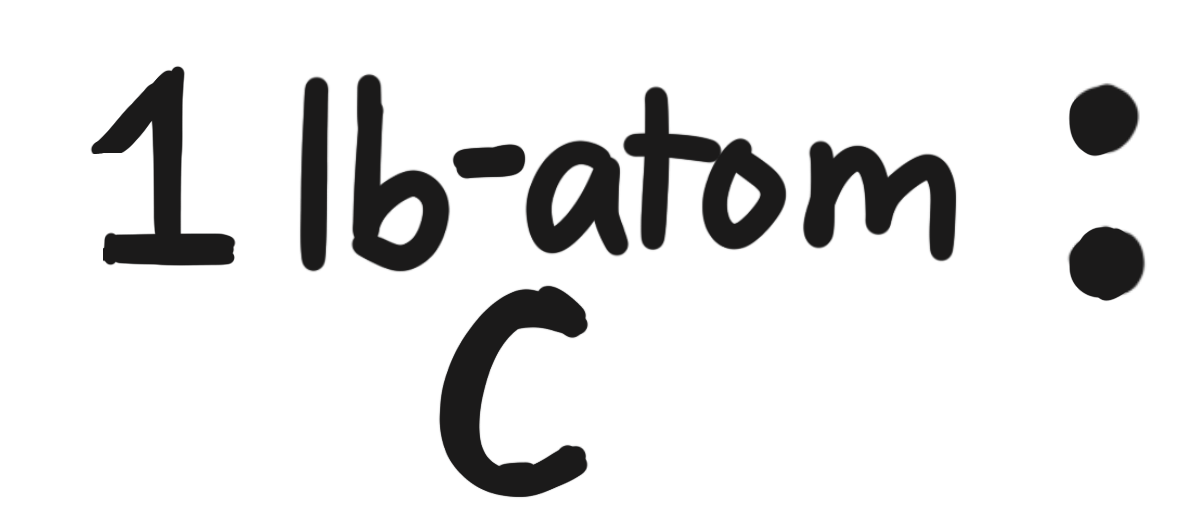
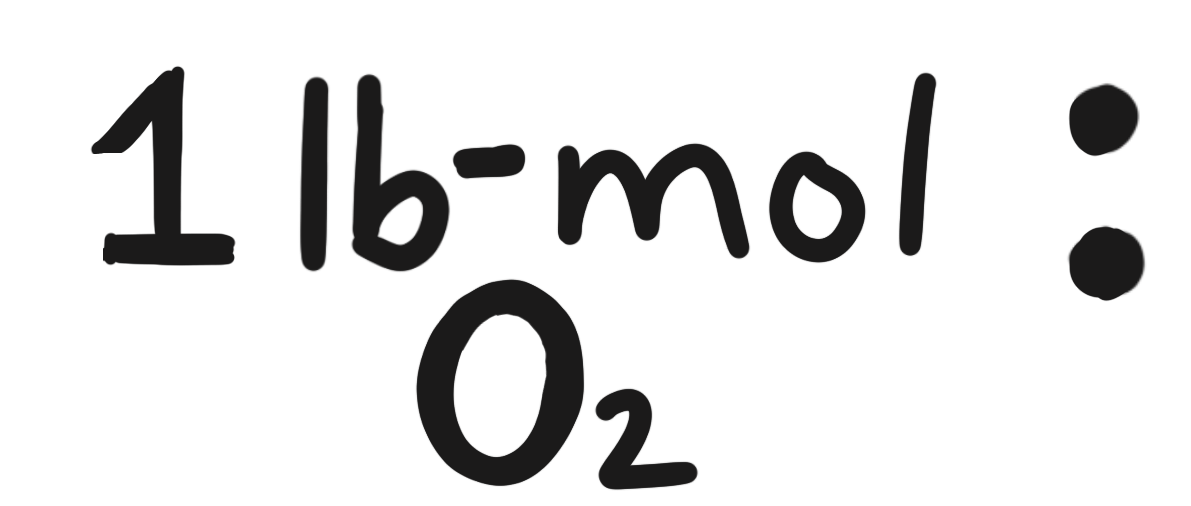
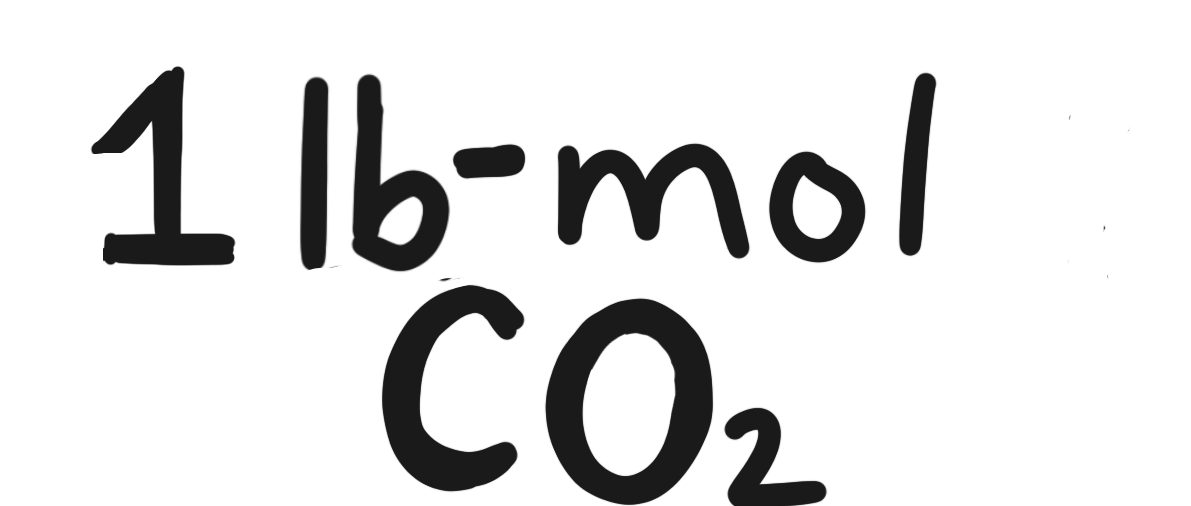
molar mass of \(\mathrm{CO_2}\) \(\rightarrow\) \(44.009546\), rounds to \(44.010\)
- \(100\ \mathrm{lb_m\ CO_2} \cdot \frac{1\text{ lb-mol }\mathrm{CO_2}}{44.010\text{ }\mathrm{lb_m}} = 2.272\ \text{lb-mol}\) of \(\mathrm{CO_2}\)
- 1:1:1 molar ratio:
- \(2.272\ \text{lb-mol C}\)
- \(2.272\ \text{lb-mol }\mathrm{O_2}\)
\(\cdot 12.011\ \frac{\mathrm{lb_m\ C}}{\text{lb-mol}} = 27.29 \mathrm{lb_m\ C}\)
\(\cdot 31.999\ \frac{\mathrm{lb_m\ O}_2}{\text{lb-mol}} = 72.70 \mathrm{lb_m\ O}_2\)
Avogadro’s Number
The Avogadro Constant/
Avogadro’s Number:
\(6.02214076 \times 10^{23}\ \frac{\mathrm{molecules}}{\mathrm{mol}}\)
\(\text{lb-mol}\) constant:
\(1.327 654 687 336 \times 10^{27}\ \frac{\mathrm{molecules}}{\text{lb-mol}}\)
Use the Correct Moles
- Unless the problem is in grams, don’t use gram moles!
- Inefficient steps for using gram moles:
- Start with mass in units other than grams.
- Convert to grams.
- Convert to gram-moles.
- Use balanced chemical equation to solve for the other species in gram-moles.
- Convert the other species’ gram-moles to grams.
- Convert the grams to the starting mass species.
- Start with mass in units other than grams.
- Requires five steps.





Use the Correct Moles
- Unless the problem is in grams, don’t use gram moles!
- Efficient steps for using moles:
- Start with mass in units other than grams.
- Convert to the mass unit’s moles.
- Use balanced chemical equation to solve for the other species in the mass unit’s moles.
- Convert the other species’ moles to mass units.
- Start with mass in units other than grams.
- Requires three steps instead of five.
- 40% fewer places to make mistakes!
The Takeaways
- Different types of moles are for different types of mass units.
- The molar mass is just the conversion factor between moles and mass. It has the same numerical value for all mass/mole pairs.
- Use gram moles for problems in grams. Use pound-moles for problems in pounds, etc.
Thanks for watching!
The Full Story companion video is in the link in the upper left. The next video in the series is in the upper right. To learn more about Chemical and Thermal Processes, visit the website linked in the description to find previous and following videos in this series.
The DOFPro Team

